
Check to see if the equation is balanced.At what temperature will this reaction be spontaneous?.Calculate the Temperature when the above reaction is at equilibrium ( DG = 0).
#Entropy and enthalpy free
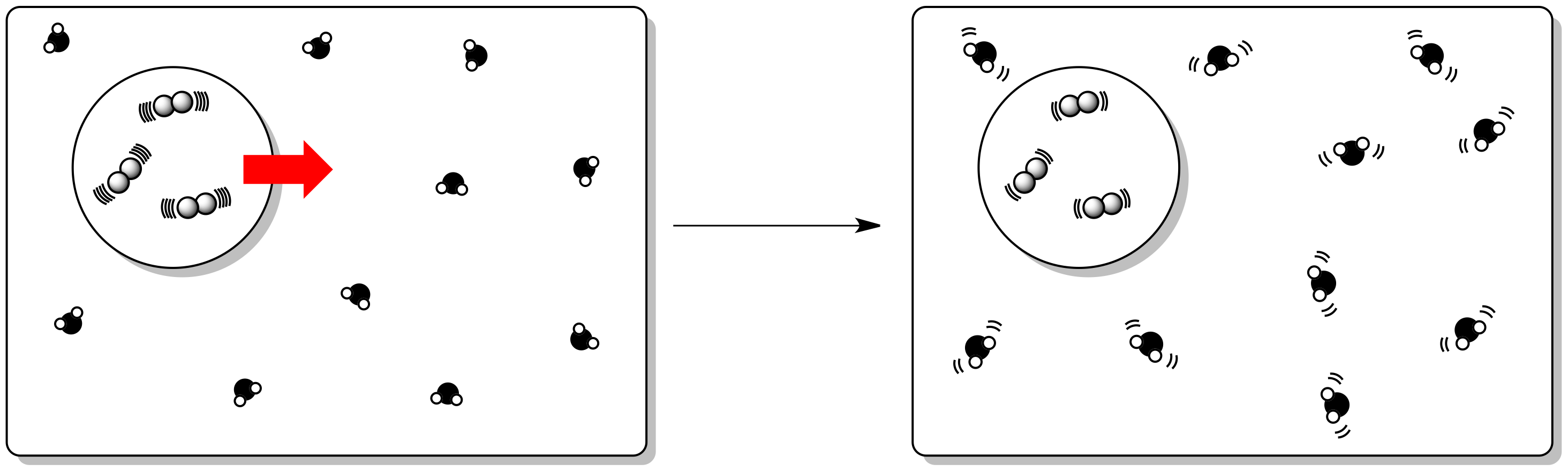
Sum of Free Energy of Products - Sum of Free Energy of Reactants = (-2052 kJ) - (484 kJ) = -1568 kJ = Standard Free Energy Change for the Reaction.ģ) For the following reaction using the Thermodynamics table: Subtract the result of step 9 from the result of step 5 to get the Standrad Free Energy Change for the Reaction.(484) + (0.00) = 484 kJ = Standard Free Energy for Reactants Add the results of steps 7 and 8 to get the Standard Free Energy of the Reactants.Look up the Standard Free Energy of Formation of O 2(g) and multiply by its coefficient(5)ĥ moles (0.00 kJ/mole) = 0.00 kJ = Standard Free Energy of 5 moles of O 2(g).Look up the Standard Free Energy of Formation for HCN(l) and multiply by its coefficient(4)Ĥ moles ( 121 kj/mole) = 484 kJ = Standard Free Energy for 4 moles HCN(l).Add the results of steps 2,3, and 4 to get the Standard Free Energy for the products.Look up the Standard Free Energy of Formation of N 2(g) and multiply by its coefficient(2)Ģ moles(0.00 kj/mole) = 0.00 kJ = Standard Free Energy of Formation for 2 moles of N 2(g).Look for the Standard Free Energy of Formation of CO 2(g) and multiply by its coefficient (4)Ĥ moles ( -394.4 kj/mole) = -1577.6 kJ = Standard Free Energy of Formation for four moles CO 2.Look up the Standard Free Energy of Formation of H 2O(g) and multiply by its coefficient(2) in the equation.Ģ moles ( -237.2 kj/mole) = -474.4 kj = Standard Free Energy of Formation for two moles H 2O(l).Check to make sure the equation is balanced.Therefore the reaction becomes spontaneous when T = 469 K (196 ✬)īelow this temperature the reaction is spontaneous.Ģ) Determine the Delta G under standard conditions using Gibbs Free Energies of Formation found in a suitable Thermodynamics table for the following reaction:ĤHCN(l) + 5O 2(g) -> 2H 2O(g) + 4CO 2(g) + 2N 2(g) If ΔG = 0 then the system is at the limit of reaction spontaneity ΔG = -93000 - (T x -198) note that the enthalpy is given in kilojoules Standard Free Energy Change, DG o -the standard free energy change, DG o can be calculated (1) by substituting standard enthalpies and entropies of reaction and a Kelvin temperature into the Gibbs equation or (2) by combining standard free energies of formation through the expression In many cases, we can predict the sign of from the signs of It is defined by the Gibbs equation:įor a spontaneous process at constant temperature and pressure, DG must be negative. The free energy change, DG is equal to -T DS univ and it applies just to a system itself, without regard for the surroundings. Total entropy change, also called the entropy change of the universe, is the sum of the entropy change of a system and of its surroundings:Īccording to the second law of thermodynamics, the entropy of the universe, S univ must always increase for a spontaneous process, that is, DS univ>0.įree Energy and Free Energy Change -the Gibbs free energy, G, is used to describe the spontaneity of a process. The direction of spontaneous change is the direction in which total entropy increases.


 0 kommentar(er)
0 kommentar(er)
